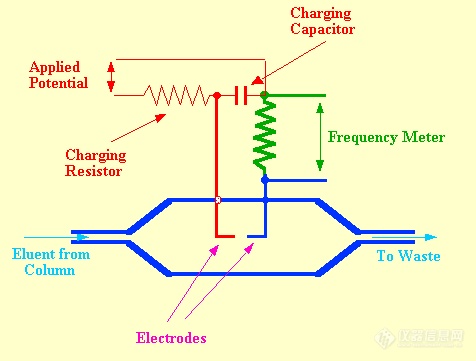
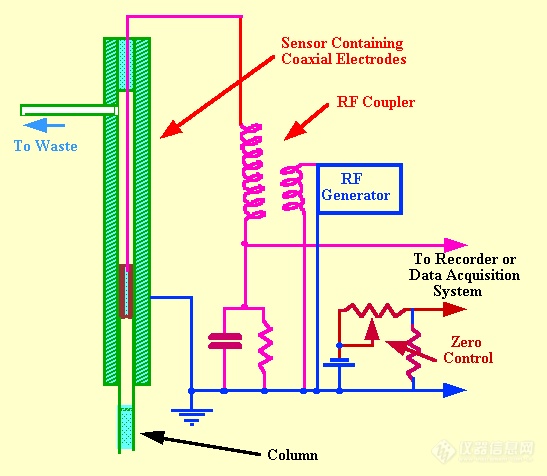
The Ultrasound Whistle DetectorThe velocity of the propagation of sound through a gas depends on its density and, thus, the presence of a solute vapor in a gas changes the velocity of sound through it. This velocity change can be utilized as a basis for vapor detection in
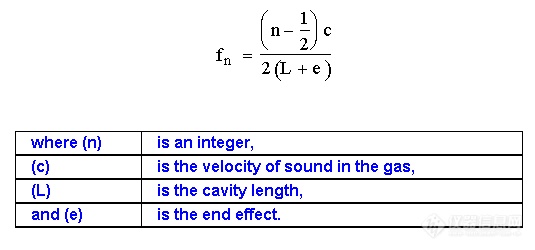
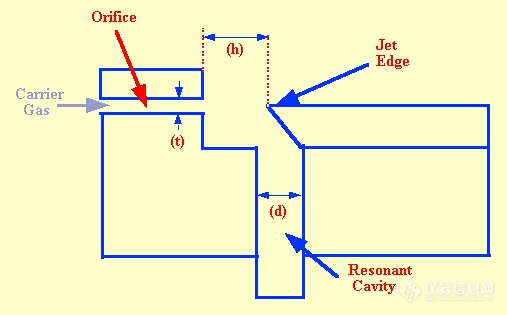
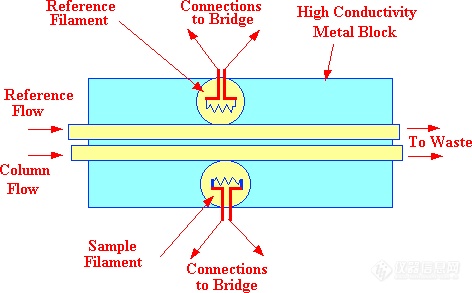
gc. The frequency of a whistle, consisting of an orifice which directs a stream of gas against a jet edge proximate to a resonant cavity, is related to the velocity of sound in the gas passing through it. A diagram of such a whistle is shown in figure 12. Nyborg et al. (38) showed that the frequency (fn) of the whistle could be described by the following equation.
![]()
Testerman and McLeod designed and built a detector based on the whistle principle. In their sensor design, typical values taken for the dimensions in the diagram, and variables in the equation, were (t), 0.064 mm, (d), 0.74 mm, (h), 1.676 mm and (L) 3.81 mm.
![]()
Under the flow conditions normally used for
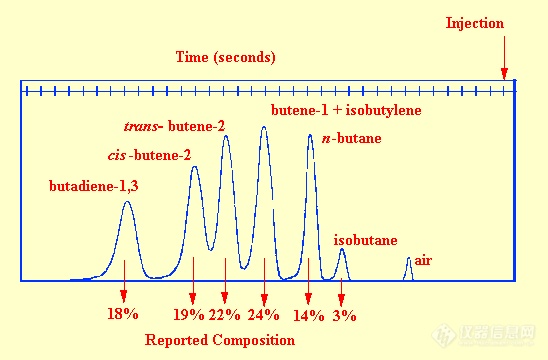
gc separations, frequencies ranging from 30-50 kHz (supersonic frequencies) were observed. The sensor contained two sound generators, one operating with pure carrier gas and the other with the eluent from the column. The two frequencies were allowed to beat together, the beat frequency being directly related to the frequency difference between the two whistles and consequently the density difference between the contents of the two sensors. An example of the use of the whistle detector to monitor the separation of a mixture of hydrocarbons is shown in figure 60. The sample size was 7.5 ml of gas mixture and the carrier gas flow rate was 180 ml /min. This chromatogram illustrates the effective use of the detector and the operating conditions shows its limitations. The sensitivity appears somewhat less than that of the katharometer but the very high flow rates necessary to activate the whistle restrict the use of this type of detector very severely. In the original report the linearity was stated to cover 2 orders of magnitude of concentration but with modern electronics it is likely that this linear range could be extended by at least another order of magnitude.
![]()
Figure 60 The Separation of a Mixture of Hydrocarbons Monitored by the Supersonic Whistle Detector