TYPES OF WATER
There are many different grades of water used for pharmaceutical purposes. Several are described in USP monographs that specify uses, acceptable methods of preparation, and quality attributes. These waters can be divided into two general types: bulk waters, which are typically produced on site where they are used; and packaged waters, which are produced, packaged, and sterilized to preserve microbial quality throughout their packaged shelf life. There are several specialized types of packaged waters, differing in their designated applications, packaging limitations, and other quality attributes.
There are also other types of water for which there are no monographs. These are all bulk waters, with names given for descriptive purposes only. Many of these waters are used in specific analytical methods. The associated text may not specify or imply certain quality attributes or modes of preparation. These nonmonographed waters may not necessarily adhere strictly to the stated or implied modes of preparation or attributes. Waters produced by other means or controlled by other test attributes may equally satisfy the intended uses for these waters. It is the user's responsibility to ensure that such waters, even if produced and controlled exactly as stated, be suitable for their intended use. Wherever the term “water” is used within this compendia without other descriptive adjectives or clauses, the intent is that water of no less purity than Purified Water be used.
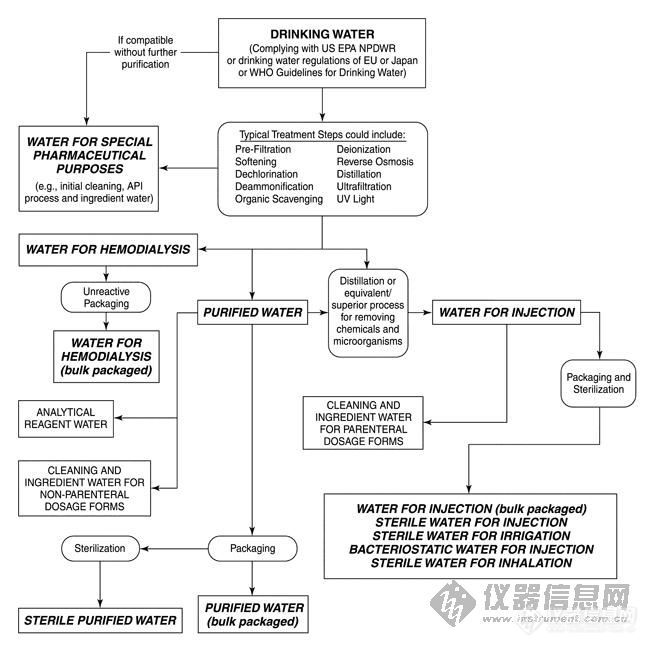
What follows is a brief description of the various types of pharmaceutical waters and their significant uses or attributes. Figure 1 may also be helpful in understanding some of the various types of waters.
Figure 1. Water for pharmaceutical purposes.
Bulk Monographed Waters and Steam
![]()