The Katharometer Detector![]()
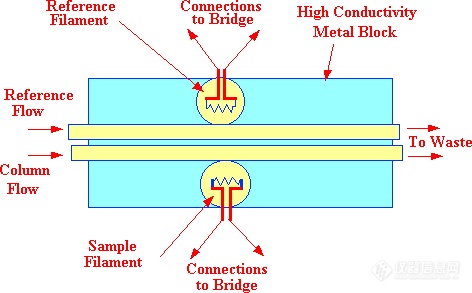
Figure 13. The Off-Line Katharometer Sensor
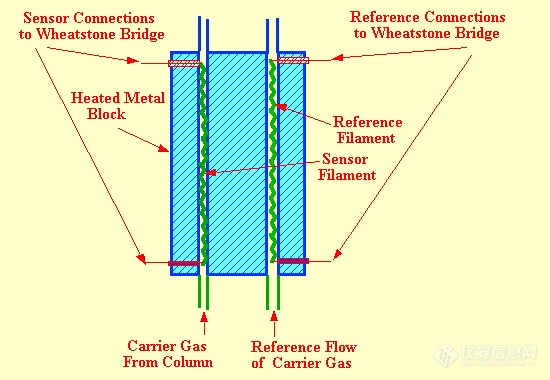
There are two types of sensor design, the "in-line" sensor where the column eluent actually passes directly over the filament (as shown in figure 12) and the "off-line" cell where the filaments are situated away from the main carrier gas stream and the gases or vapors only reach the sensing element by diffusion.(as shown in figure 13). Due to the high diffusivity of vapors in gases, the diffusion process can be considered as almost instantaneous. The filament wire is usually made from tungsten or platinum as both metals have high temperature coefficients of resistance and at the same time are relatively inert. The column and reference filaments are situated in the arms of a Wheatstone Bridge and a suitable current is passed through the filaments to heat them significantly above ambient temperature. To ensure temperature stability, the sensors and their conduits are installed in a high thermal conductivity metal block which is thermostatted by means of a separate oven. The performance of the in-line sensor is almost identical to that of the off-line sensor.
For maximum sensitivity hydrogen or helium is used as the carrier gas. The katharometer sensitivity is only about 10-6 g/ml (probably the least sensitive of all
gc detectors) and has a linear dynamic range of about 500 (the response index being between 0.98 and 1.02).
![]()
Figure 14. The Separation of the Compounds of Hydrogen, Deuterium and Tritium
Despite its sensitivity shortcomings the katharometer can be used in most
gc analyses that utilize packed columns and where there is no limitation in sample availability. The device is simple, reliable, rugged and relatively inexpensive. An example of the use of a katharometer to monitor the separation of various compounds of hydrogen, deuterium and tritium, employinggas solid chromatography is shown in figure 14. The stationary phase was activated alumina [treated with Fe(OH)2], and the column was 3 m long and 4 mm I.D. The carrier gas was neon, the flow rate 200 ml/min (at atmospheric pressure) and the column temperature was -196oC.