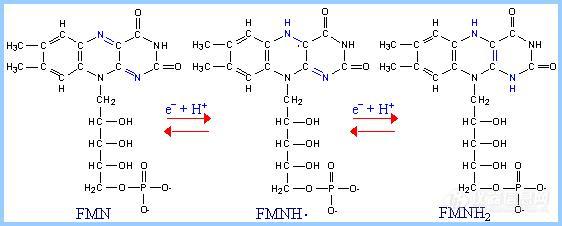
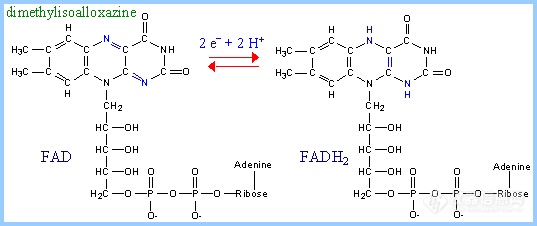
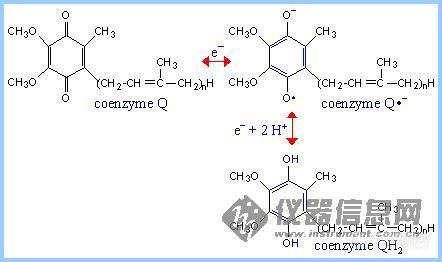
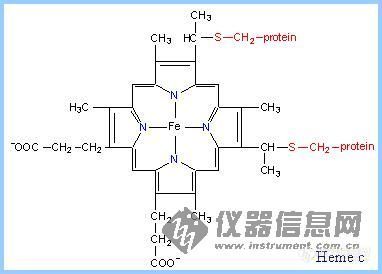
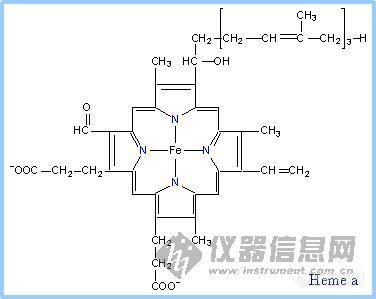
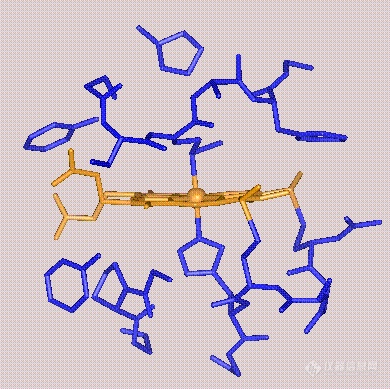
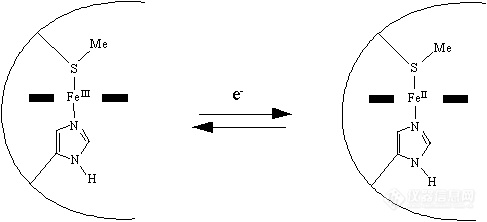
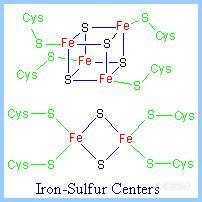
前言:电子的转移和传递是实现各种电化学生物传感器的机理所在.基于此,我们可以将我们所做的工作简单归纳为:选用或合成合适的电子转移和传递材料,通过合适的组装和组合形式,得到具有理想功能的器件.这样的材料有很多,这里仅列出一些在生物传感器研究中常见具有电子转移功能的生物分子.
1.
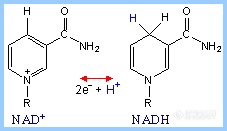
NAD+/ NADHNAD+ (Nicotinamide Adenine Dinucleotide) functions as an electron acceptor in catabolic pathways.
The nicotinamide ring of NAD+, which is derived from the vitamin niacin, accepts 2 e- and one H+ (a hydride) in going to the reduced state, as NAD+ becomes NADH
![]()
The electron transfer reaction may be summarized as:
NAD+ + 2 e- + H+ <-> NADHIt may also be written as:
NAD+ + 2 e- + 2H+ <-> NADH + H+