9. Contamination From Reagents
High Purity Water
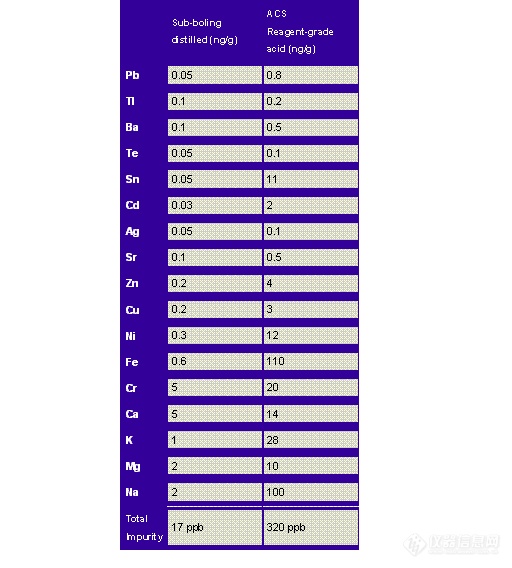
Sample preparations using acid digestion, fusion, or ashing all typically use water as the primary reagent. Most water used in trace metal laboratories is produced by systems that use ion-exchange purification. This water is commonly referred to as "conductivity water" because its conductivity approaches the theoretical conductivity of water (0.055 microhm / cm {18.2 megohm water} at 25°C).
Under normal laboratory conditions, conductivity water never measures to be 18.2 megohm due to the presence of CO2 (H2CO3HCO3- + H+). Furthermore, if you could find water giving a conductivity of 18.2 megohm, it is not necessarily free of trace elemental contaminants because only ionized compounds are detected by conductivity measurement.
Through carefully controlled experiments and measurements in clean room facilities, we have found that conductivity water will typically give readings closer to 16 megohm and that it is free of trace metallic impurities down to conventional
ICP-MS and axial view ICP-OES detection limits. We have also found that sub-ppb level impurities that were once thought to be coming from the water, are in actuality from the atmosphere and the container materials (see earlier parts of this guide). We have found that the use of clean room facilities and high temperature nitric acid-leached LDPE bottles are necessary for reliably measuring common contaminant elements in water. Therefore, do not assume that your water has significant levels of elemental contaminants if it gives conductivity readings between 16 and 17 megohm and your
ICP-MS or OES is detecting trace levels of the common environmental contaminants.
Storage of High Purity Water
High purity water should be used ASAP. "Stored" high purity water may pick up impurities from the storage container. Popular storage containers are made from quartz, polyethylene (both high and low density), and fluoropolymers.
Quartz:
Quartz (fused quartz or vitreous silica) typically contains 98.8% SiO2, and impurities consisting mainly of Na2O, Al2O3, Fe2O3, MgO, and TiO2. Quartz has a solubility in water of 11 ppm.1 We have measured a solubility of quartz in conductivity water of 11.2 ppm as silicic acid (equilibration time is ~4 weeks using 400 mesh quartz powder). [ A Closer Look at Quartz ]
HDPE and LDPE:
A significant amount of HDPE is manufactured using alumina / silica based catalysts. Long term storage in high density polyethylene (HDPE) can result in ppm levels of Ca, Mg, Si, Ti, Al and ppb levels of Cr, V and Fe. LDPE can be manufactured using an organic catalyst. Storage in HNO3 leached LDPE is optimum. Through study, we've discovered that short term (1-5 days) storage in both 20 liter HDPE and LDPE cubi containers that have been leached with dilute HNO3 do not leach any elements at
ICP-MS / OES detection limits.
Fluoropolymers:
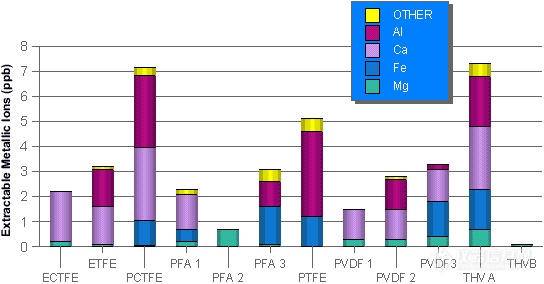
Fluoropolymers are not as clean as generally thought (see below figures). Studies performed in our own laboratories confirm these results. It is our recommendation that you save your money and use LDPE.