13. Sample Preparation by Fusion
This section will deal with fusions for trace analysis using inorganic fluxes. These techniques are required for sample types that are inorganic in nature and unreactive toward acid decomposition.
Useful Fusions for Trace Analysts
Fusions are considered to be more of a 'last resort' by trace analysts because:
They are expensive and often not available (high purity fluxes).
They yield high solids solutions that can salt out in the nebulizer.
Large dilutions of the sample are a necessity.
They often require expensive equipment.
Spectral interferences from the flux and/or crucible construction material must be considered.
Contamination of the sample with the crucible construction element and impurities must be considered.
They are labor intensive.
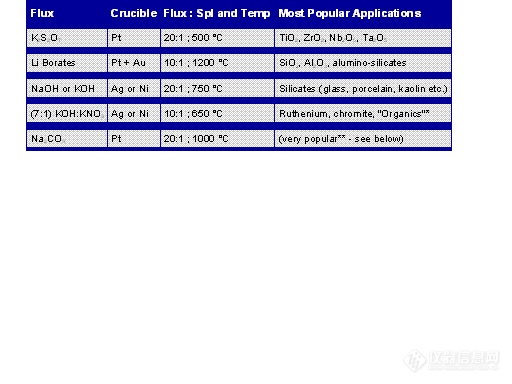
However I have found it necessary and even advantageous at times to perform a wide variety of fusions. Table 13.1 provides a summary of those that I have found to be most useful:
Table 13.1: Recommended Fusions
![]()
* "Organics" refers to organic containing samples. Both the sodium and potassium hydroxide/nitrate mixtures have been used for a wide variety of biological materials, soils, coal, and organic samples.
** Na2CO3 fusions are very popular in the decomposition of minerals, silicates, refractories, insoluble metal fluorides, etc. For example, brookite TiO2 will not be attacked by acids, and Al2O3 is very resistant to acid attack.